Cubic zinc sulfide (ZnS) is a well-known II–VI semiconductor material that has attracted great attention due to its wide band gap, excellent infrared transmission, and unique luminescence properties. It crystallizes in the zinc blende structure, a prototype crystal form for many other semiconductors. Because of its combination of optical transparency, high electronic quality, and structural stability, cubic ZnS is widely regarded as a material with significant potential in semiconductors, infrared optics, and advanced optoelectronic devices.
What is Cubic Zinc Sulfide?
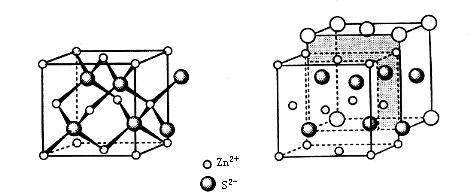
Cubic zinc sulfide is one form of ZnS crystal, composed of sulfur and zinc atoms that each form a face-centered cubic lattice. The two lattices interpenetrate with a relative displacement of one-quarter of the body diagonal. In this crystal structure, sulfur atoms are arranged in cubic close packing, and zinc atoms occupy half of the tetrahedral voids formed by the sulfur atoms. Cubic ZnS is the most representative compound with the zinc blende structure; therefore, the zinc blende structure is also known as the cubic ZnS structure.
Zinc sulfide (ZnS) is an inorganic compound that can be used as a semiconductor material, infrared optical material, and phosphor. ZnS is a group II–VI intrinsic semiconductor with a wide band gap and excellent optical properties. It shows low dispersion in the infrared region and exhibits fluorescence and electroluminescence, making it suitable for applications such as infrared windows, lasers, sensors, and flat-panel displays.
Crystal Structure of ZnS
Zinc sulfide exists in two polymorphs: the α-phase (wurtzite structure, hexagonal α-ZnS) and the β-phase (zinc blende structure, cubic β-ZnS). The cubic phase is stable at lower temperatures, while the hexagonal phase is the high-temperature modification. Heating cubic ZnS can lead to a phase transformation into hexagonal ZnS. Naturally, ZnS is commonly found in the zinc blende form, which makes it abundant in mineral resources. Importantly, cubic ZnS has a band gap of about 3.66 eV, wider than many conventional semiconductors, making it suitable for applications that require high transparency in the visible and infrared regions.
Synthesis Methods
A variety of approaches have been developed to obtain the zinc blende phase of ZnS with controlled morphology and high phase purity:
- Hydrothermal synthesis: This technique enables the growth of high-quality crystals and hollow microspheres. The resulting materials often display photoluminescence in the blue and green regions, which is valuable for optical applications.
- Microwave-assisted hydrothermal synthesis: By combining solid-state chemistry with microwave irradiation, researchers can rapidly produce monodisperse nanostructures that exhibit enhanced catalytic and optical activity.
- Solid-state synthesis: Pure cubic-phase ZnS can be prepared even at room temperature, showing a blue-shifted absorption edge and excellent infrared transparency.
- Vapor-phase methods: Techniques such as chemical vapor deposition (CVD) and chemical vapor transport (CVT) are frequently employed to grow thin films and single crystals, making them particularly suitable for electronic and optoelectronic devices.
Compounds with Zinc Blende Structure
The zinc blende structure is widely observed in many technologically important materials. III–V semiconductors such as gallium arsenide (GaAs), indium antimonide (InSb), gallium phosphide (GaP), and gallium antimonide (GaSb) all crystallize in this form. Other examples include cubic boron nitride (c-BN), cubic silicon carbide (β-SiC), and copper(I) halides such as CuCl, CuBr, and CuI. The structural similarity between these compounds and cubic ZnS often translates into comparable physical and electronic behavior, which is crucial for semiconductor device design.
Advantages
III–V compounds are important semiconductor materials, and cubic Zinc sulfide, as a II–VI semiconductor, shares the same zinc blende structure while offering a wider band gap. Compared with hexagonal ZnS, cubic ZnS has a slightly narrower band gap of 3.66 eV, but it is still significantly larger than that of common semiconductors such as GaAs and SiC. Moreover, phase-pure cubic ZnS without hexagonal inclusions exhibits excellent infrared transmittance. Overall, cubic zinc sulfide holds great potential in the fields of semiconductors and infrared optics.
Cubic zinc sulfide (ZnS) is not only an abundant mineral form of zinc but also an advanced semiconductor material with exceptional optical and electronic properties. With its zinc blende crystal structure, wide band gap, and excellent infrared transmission, it represents a promising material for semiconductors, infrared optics, and next-generation optoelectronic devices.
